
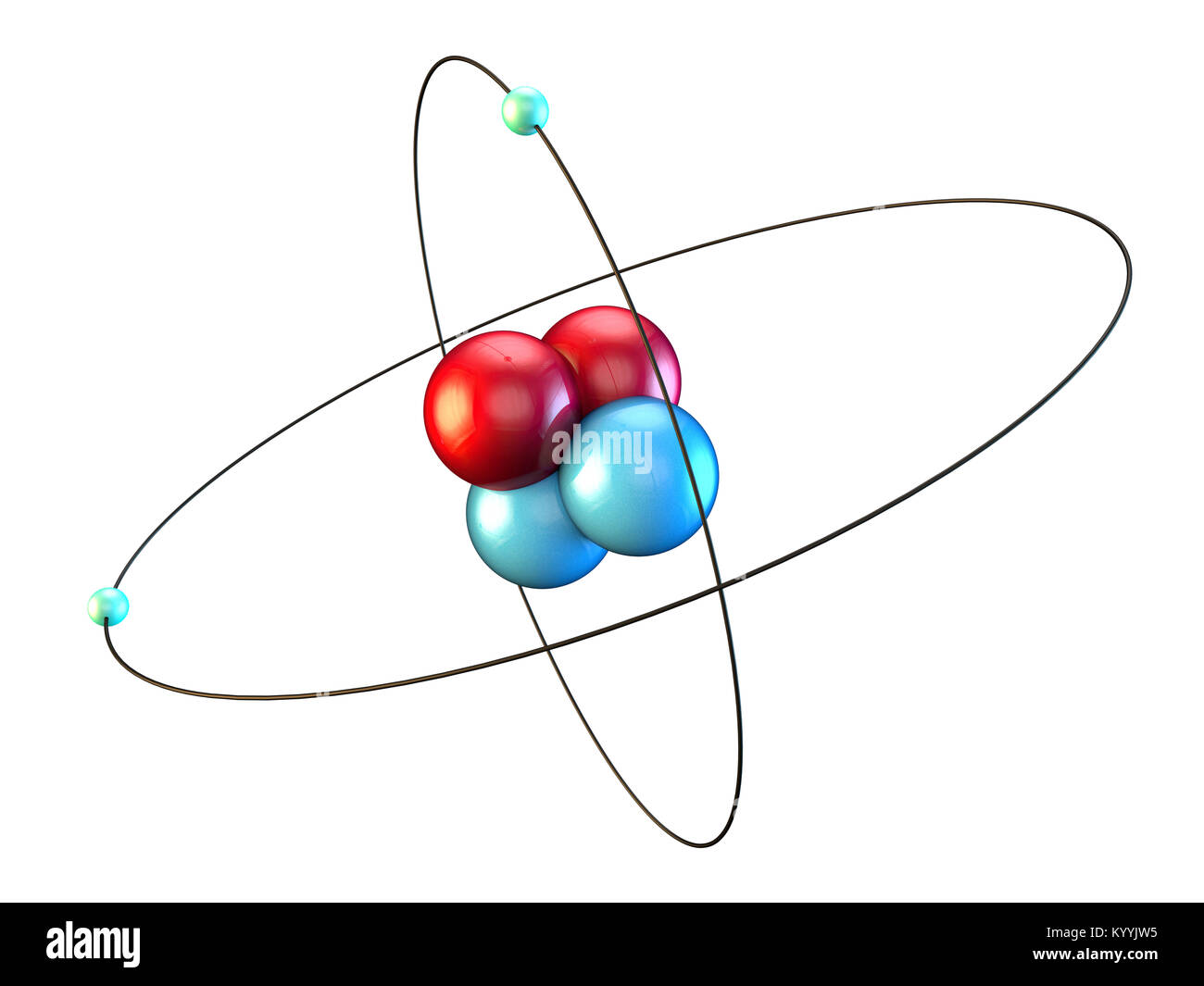
In general, strong or polar bonds are the result of the electron cloud of one atom being drawn towards the electron cloud of another atom, such that the negative or positive charge of the electron clouds increases. These behaviors merge into each other seamlessly in various circumstances, so that there is no clear line to be drawn between them. This attraction may be seen as the result of different behaviors of the outermost or valence electrons of atoms. A chemical bond is an attraction between atoms. Many types of bond exist between atoms, such as single, double, and triple bonds and bonds of particular strength, including ionic and covalent bonds. The strength of chemical bonds between atoms ranges from the weakest to the strongest. The bond may result from the electrostatic force of attraction between opposite charges, dipole–dipole attraction (see also polarization (chemistry)), or the sharing of electrons as in covalent bonding. In chemistry, a chemical bond is a lasting attraction between atoms that enables the formation of chemical compounds. And if we compare this with the value that is mentioned in the periodic table, uh this mhm value. Times 6.2 times 10 to the 23 atoms Present in one mole. We would have 6.64, 2 times 10 to the -24 g per adam. So this would be a moller mouse still to calculate the molar mouse. And for part d calculate the mouse of a mole of these helium atoms. This would give us yeah 6.6 4, 2 times 10 to the -24 g. Here, one Atomic mass unit Is equal to 166054, 10 to the -24g. For part C calculate the mass of the atom in grams. Still, This would total up to be four atomic mass units. Two neutrons even would have to atomic mass units and two electrons in terms of atomic mass units to be zero. So two protons mhm would have two Atomic mass units. For part B, calculate the mass of the atom in unified atomic mass units. Two electrons are outside of the nucleus. Okay, two protons and two neutrons Mhm are present in the nucleus. For a explain how these particles are arranged to make the atom so in a huge liam adam. Little that helium has two protons, Two electrons and two neutrons. In general, strong or polar bonds are the result of the electron cloud of one atom being drawn towards the electron cloud of another atom, such that the negative or positive charge of the electron clouds increases


 0 kommentar(er)
0 kommentar(er)
